We fund collaborative research to bring faster cures to patients
The SWCRF pioneered the practice of collaboration among cancer researchers across the world. Our funded researchers share their findings to accelerate the pace and impact of new discoveries and promising cures.
Our philosophy of collaboration has built a scientific brain trust with global reach. Once investigators receive funding from the SWCRF, they become members of our Institute Without Walls™, a world-class group of 50 scientists, physicians, and oncologists who share information and resources to speed the pace of cancer research.
Our commitment to collaboration has resulted in significant breakthroughs—from discovering genetic mutations that cause cancer to identifying promising new therapies. Grants to SWCRF-funded scientists often leverage additional funding from major cancer organizations, such as the National Cancer Institute.
In addition to supporting ongoing collaborative research on specific cancers, our scientists are investigating the biology of cancer to find treatments across disease types.
This collaborative model of scientific investigation has led to several discoveries that have been published in the world's leading scientific journals.
HOW WE INVEST IN CANCER RESEARCH
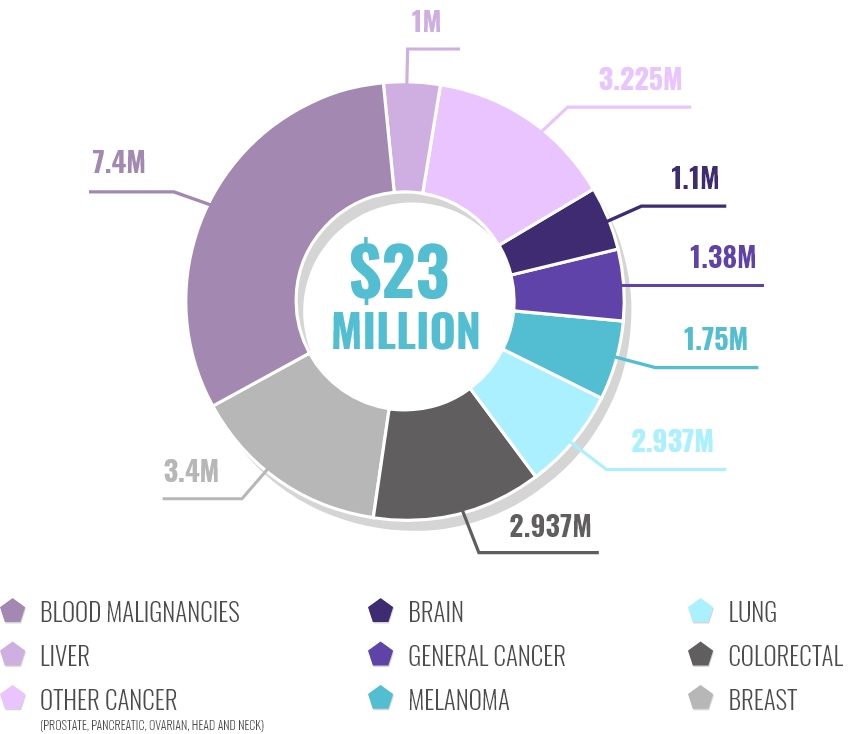
OUR ACHIEVEMENTS THROUGH
COLLABORATION
Research we funded created a new combination of two existing cancer therapies for the treatment of hormone-resistant breast cancer, producing a new clinical trial at several New York hospitals.
LIVER CANCER
Our funded research pioneered the use of the first successful liver cancer drug treatment through an international consortium.
LEUKEMIA
We partnered with researchers from the Shanghai Institute of Hematology to develop the first successful targeted differentiation therapy for acute promyelocytic leukemia (APL) with all-trans-retinoic acid and arsenic trioxide which increased the disease’s five-year survival rate from 25% to 95%.
PEDIATRIC CANCER
We brought together scientists from the Dana-Farber Cancer Institute to develop a targeted therapy for rare childhood cancer, which is currently in clinical trials.
LUNG CANCER
We advanced the paradigm of treatment for lung cancer by adding targeted drugs to chemotherapy through a collaboration between the Dartmouth Cancer Center and Tisch Cancer Institute at Mount Sinai